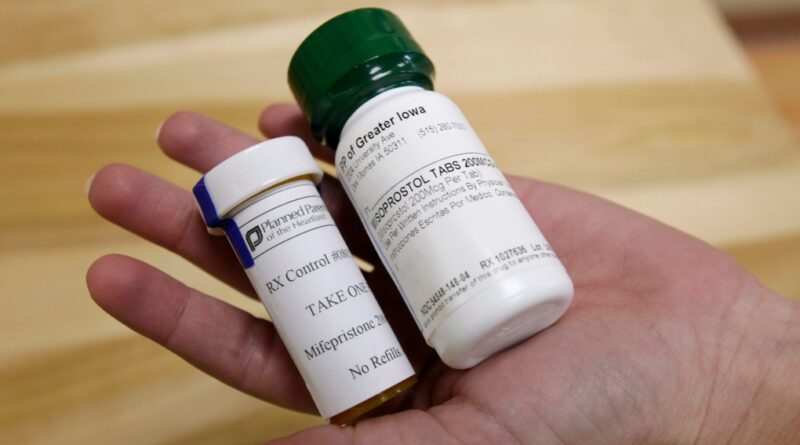
Hyundai Pharma’s upcoming abortion drug to be named as “Mifegymiso”
Hyundai Pharma said the name of the impending abortion drug it intends to supply in Korea would be “Mifegymiso.”
Prior on Tuesday a week ago, the organization said it secured a selective concurrence with Linepharma International to sell an oral abortion medication in the homegrown market.
Mifegymiso is a mix medication of mifepristone and misoprostol. Mifepristone is known by its image name Mifegyne in Korea.
Hyundai Pharma said it chose to supply the abortive medication to help ladies access safe medicine end of pregnancy.
The Ministry of Food and Drug Safety is purportedly leading a starter audit on Mifegymiso.
The primer survey includes evaluating the wellbeing and viability information, before the organization’s accommodation of the medication endorsement application. The fundamental assessment decreases the experimentation during the endorsement audit measure, in this way shortening the time needed for the advertising endorsement.
The Association of Pharmaacists for Healthy Society (APHS), which has been pushing for the presentation of a fetus removal medication in Korea, invited Hyundai Pharma’s arrangement to carry out Mifegymiso. It additionally said that the controller ought to turn out to be more dynamic in conceding the gesture for the medication.
Lee Dong-geun, secretary-general of the APHS, noticed that the food and medication security service in January said it would think about taking a sped up endorsement strategy if a drug organization proposed to supply a fetus removal drug in Korea.
“As a neighborhood drugmaker is finding a way ways to present a failed drug, it is currently the ideal opportunity for the service to stay faithful to its obligation,” he said.
At the point when the wellbeing specialists set rules for the utilization of fetus removal prescription, they ought to focus on ladies’ “openness” to the medication, Lee said.
“Women need an abortion pill because they can get better access to the drug when they need to discontinue pregnancy. But in small cities, it is not even easy to visit an obstetrician/gynecologist,” he said.
The public authority ought to permit subject matter experts and general professionals to endorse an early termination drug for outpatients without hospitalization, he added.
For more latest news Click Here
Follow Us
Twitter: @TimesTechPharm1