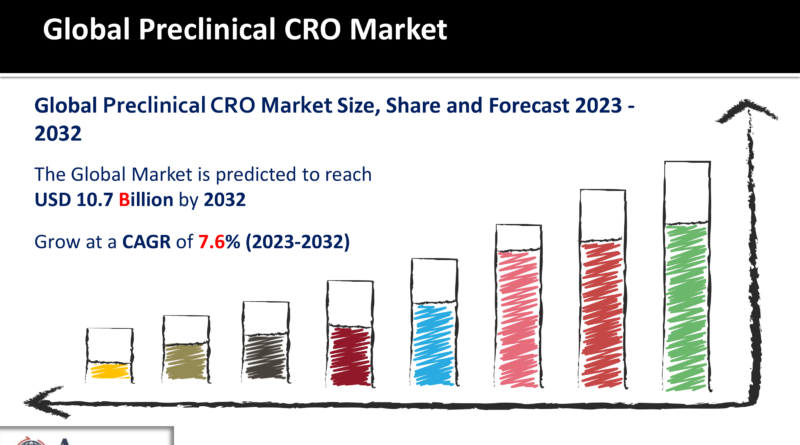
Preclinical CRO Market Size to touch USD 10.7 Billion by 2032
The Preclinical CRO Market Size made up for USD 5.2 Billion in 2022 and is estimated to reach a market cap USD 10.7 Billion by 2032 expanding at 7.6% CAGR, between 2023 and 2032.
Introduction
The preclinical Contract Research Organization (CRO) market is currently experiencing significant growth, driven by a confluence of factors that present both opportunities and challenges for industry players. In this article, we will delve into the dynamics of the preclinical CRO market, examining the latest trends, market drivers, restraints, regional insights, the competitive landscape, and future growth prospects.
Download Free Preclinical CRO Market Sample Report Here: (Including Full TOC, List of Tables & Figures, Chart)https://www.acumenresearchandconsulting.com/request-sample/2480
 Market Trends
Market Trends
The preclinical CRO market has witnessed several noteworthy trends in recent years. One of the most prominent trends is the growing emphasis on outsourcing by pharmaceutical and biotechnology companies. As these companies aim to reduce development costs and expedite drug discovery processes, they increasingly turn to CROs for their preclinical research needs.
Additionally, the industry is witnessing a surge in demand for specialized preclinical services. With the development of innovative therapies, such as gene and cell therapies, and an increasing focus on precision medicine, there is a rising demand for CROs that possess expertise in niche areas, such as genomics, toxicology, and bioinformatics.
Press Release:https://www.acumenresearchandconsulting.com/press-releases/preclinical-cro-market
Market Drivers
Several drivers are propelling the preclinical CRO market forward. First and foremost, the global pharmaceutical industry’s robust growth is a key driver. As the need for novel therapeutics continues to rise, pharmaceutical companies are seeking the expertise of CROs to conduct efficient and comprehensive preclinical testing.
Moreover, the current regulatory environment emphasizes thorough preclinical testing to ensure the safety and efficacy of new drugs. This regulatory focus is stimulating demand for CROs that specialize in conducting preclinical trials in compliance with stringent regulatory standards.
Market Restraints
While the preclinical CRO market shows immense promise, it is not without its challenges. Pricing pressures are one of the primary restraints affecting the industry. As pharmaceutical and biotechnology companies seek cost-effective solutions, CROs must find ways to provide high-quality services while maintaining competitive pricing.
Furthermore, the complexity and variability of preclinical research can be a hindrance. Researchers often encounter issues related to data reproducibility, which can slow down the drug development process. Addressing these challenges requires ongoing investment in technology and talent.
Opportunities in the Preclinical CRO Market
Despite the challenges, the preclinical CRO market offers abundant opportunities for growth and innovation. The expansion of the biopharmaceutical sector, especially in emerging markets, presents a significant opportunity for CROs to tap into new geographies. The Asia-Pacific region, in particular, is emerging as a hotspot for preclinical research outsourcing.
Moreover, advances in technologies such as artificial intelligence (AI) and machine learning are transforming preclinical research. CROs that can harness the power of these technologies to enhance their services will be well-positioned for success in this evolving market.
Regional Insights
The preclinical CRO market is not homogenous but rather displays regional variations. North America, with its established pharmaceutical industry and robust regulatory framework, remains a dominant player in the global market. Europe is also a significant market, characterized by stringent regulatory standards.
In recent years, Asia-Pacific has emerged as a high-growth region due to its lower operational costs and a growing pool of skilled researchers. With increased investments in research and development by both local and international pharmaceutical companies, Asia-Pacific is becoming a focal point for preclinical research services.
Competitive Landscape
The preclinical CRO market is highly competitive, with numerous companies vying for market share. Some of the leading players in the industry include Charles River Laboratories, Laboratory Corporation of America Holdings, Eurofins Scientific, and Pharmaceutical Product Development (PPD). These companies have a global presence and offer a wide range of preclinical research services.
In addition to the established players, niche CROs that specialize in particular areas of preclinical research, such as in-vitro toxicology or pharmacokinetics, are also gaining prominence. This diversification allows clients to find CROs with expertise tailored to their specific needs.
Future Growth Prospects
The future of the preclinical CRO market looks promising. As pharmaceutical and biotechnology companies continue to innovate and expand their pipelines, the demand for preclinical research services is expected to grow. Additionally, the integration of cutting-edge technologies, such as AI and data analytics, will enable CROs to offer more efficient and accurate services.
Furthermore, the increasing trend of collaborations and partnerships between CROs and drug developers is likely to stimulate market growth. These collaborations allow CROs to be involved in drug development projects from the early stages, increasing their importance in the drug discovery process.
Get Discount On The Purchase Of This Report:https://www.acumenresearchandconsulting.com/buy-now/0/2480
Find more such market research reports on our website or contact us directly
Write to us at sales@acumenresearchandconsulting.com
Call us on +918983225533
or +13474743864