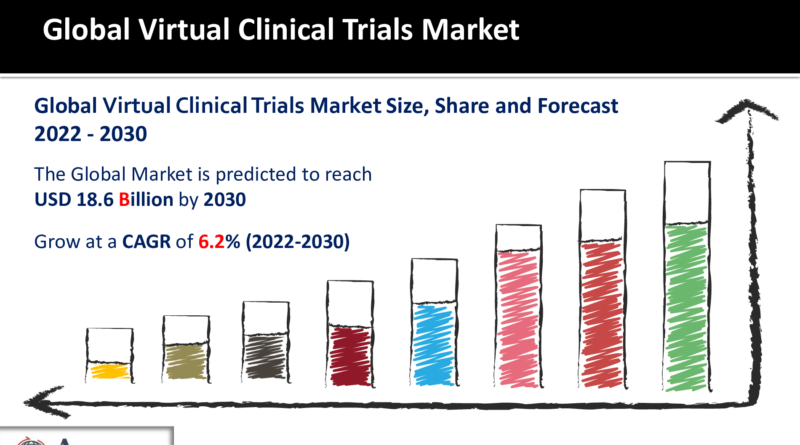
Virtual Clinical Trials Market Size to touch USD 18.6 Billion by 2030
The report analyzes and forecasts the Virtual Clinical Trials Market at global and regional levels. The market has been forecast based on volume (Tons) and value (US$ Mn) from 2022 to 2030. The study includes drivers and restraints of the global market. It covers the impact of these drivers and restraints on the demand during the forecast period. The report also highlights opportunities in the market at the global level.
The report comprises a detailed value chain analysis, which provides a comprehensive view of the global Virtual Clinical Trials Market. The Porter’s Five Forces model has also been included to help understand the competitive landscape of the market. The study encompasses market attractiveness analysis, wherein various applications have been benchmarked based on their market size, growth rate, and general attractiveness.
The study provides a decisive view of the Virtual Clinical Trials Market by segmenting it in terms of form and application. The segment has been analyzed based on the present and future trends. Regional segmentation includes the current and projected demand in North America, Europe, Asia Pacific, Latin America, and Middle East & Africa.
The report provides size (in terms of volume and value) of Virtual Clinical Trials Market for the base year 2020 and the forecast between 2021 and 2028. Market numbers have been estimated based on form and application. Market size and forecast for each application segment have been provided for the global and regional market.
Download Sample Report Copy From Here:https://www.acumenresearchandconsulting.com/request-sample/2614
In-depth interviews and discussions were conducted with several key market participants and opinion leaders to compile the research report. Primary research represents a bulk of research efforts, supplemented by extensive secondary research. Annual reports, press releases, and relevant documents of key players operating in various application areas have been reviewed for competition analysis and market understanding. Secondary research also includes recent trends, technical writing, Internet sources, and statistical data from government websites, trade associations, and agencies. These have proved to be reliable, effective, and successful approaches for obtaining precise market data, capturing market participants’ insights, and recognizing business opportunities.
 Market Players as below:
Market Players as below:
Some of the key virtual clinical trials companies in the market are ICON plc, Parexel International, IQVIA, Covance Inc., PRA Health Sciences, LEO Innovation Lab, Medidata Solutions, Oracle Corporation, CRF Health, Clinical Ink, Inc., Medable, Inc., and among others.
The major market segments of Virtual Clinical Trials Market are as below:
Virtual Clinical Trials Market By Study Design
- Interventional
- Observational
- Expanded Access
Virtual Clinical Trials Market By Indication
- Oncology
- Cardiovascular
- Other Indications
Ask Query Here: richard@acumenresearchandconsulting.com or sales@acumenresearchandconsulting.com
Table Of Contents:
CHAPTER 1. Industry Overview of Virtual Clinical Trials Market
1.1. Definition and Scope
1.1.1. Definition of Virtual Clinical Trials
1.1.2. Market Segmentation
1.1.3. Years Considered for the Study
1.1.4. Assumptions and Acronyms Used
1.1.4.1. Market Assumptions and Market Forecast
1.1.4.2. Acronyms Used in Global Virtual Clinical Trials Market
1.2. Summary
1.2.1. Executive Summary
1.2.2. Virtual Clinical Trials Market By Study Design
1.2.3. Virtual Clinical Trials Market By Indication
1.2.4. Virtual Clinical Trials Market By Region
CHAPTER 2. Research Approach
2.1. Methodology
2.1.1. Research Programs
2.1.2. Market Size Estimation
2.1.3. Market Breakdown and Data Triangulation
2.2. Data Source
2.2.1. Secondary Sources
2.2.2. Primary Sources
CHAPTER 3. Market Dynamics And Competition Analysis
3.1. Market Drivers
3.1.1. Driver 1
3.1.2. Driver 2
3.2. Restraints and Challenges
3.2.1. Restraint 1
3.2.2. Restraint 2
3.3. Growth Opportunities
3.3.1. Opportunity 1
3.3.2. Opportunity 2
3.4. Porter’s Five Forces Analysis
3.4.1. Bargaining Power of Suppliers
3.4.2. Bargaining Power of Buyers
3.4.3. Threat of Substitute
3.4.4. Threat of New Entrants
3.4.5. Degree of Competition
3.5. Market Concentration Ratio and Market Maturity Analysis of Virtual Clinical Trials Market
3.5.1. Go To Market Strategy
3.5.1.1. Introduction
3.5.1.2. Growth
3.5.1.3. Maturity
3.5.1.4. Saturation
3.5.1.5. Possible Development
3.6. Technological Roadmap for Virtual Clinical Trials Market
3.7. Value Chain Analysis
3.7.1. List of Key Manufacturers
3.7.2. List of Customers
3.7.3. Level of Integration
3.8. Regulatory Compliance
3.9. Competitive Landscape, 2021
3.9.1. Player Positioning Analysis
3.9.2. Key Strategies Adopted By Leading Players
CHAPTER 4. Virtual Clinical Trials Market By Study Design
4.1. Introduction
4.2. Virtual Clinical Trials Revenue By Study Design
4.2.1. Virtual Clinical Trials Revenue (USD Billion) and Forecast, By Study Design, 2018-2030
4.2.2. Interventional
4.2.2.1. Interventional Market Revenue (USD Billion) and Growth Rate (%), 2018-2030
4.2.3. Observational
4.2.3.1. Observational Market Revenue (USD Billion) and Growth Rate (%), 2018-2030
4.2.4. Expanded Access
4.2.4.1. Expanded Access Market Revenue (USD Billion) and Growth Rate (%), 2018-2030
CHAPTER 5. Virtual Clinical Trials Market By Indication
5.1. Introduction
5.2. Virtual Clinical Trials Revenue By Indication
5.2.1. Virtual Clinical Trials Revenue (USD Billion) and Forecast, By Indication, 2018-2030
5.2.2. Oncology
5.2.2.1. Oncology Market Revenue (USD Billion) and Growth Rate (%), 2018-2030
5.2.3. Cardiovascular
5.2.3.1. Cardiovascular Market Revenue (USD Billion) and Growth Rate (%), 2018-2030
5.2.4. Other Indications
5.2.4.1. Other Indications Market Revenue (USD Billion) and Growth Rate (%), 2018-2030
CHAPTER 6. North America Virtual Clinical Trials Market By Country
6.1. North America Virtual Clinical Trials Market Overview
6.2. U.S.
6.2.1. U.S. Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
6.2.2. U.S. Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
6.3. Canada
6.3.1. Canada Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
6.3.2. Canada Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
6.4. North America PEST Analysis
CHAPTER 7. Europe Virtual Clinical Trials Market By Country
7.1. Europe Virtual Clinical Trials Market Overview
7.2. U.K.
7.2.1. U.K. Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
7.2.2. U.K. Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
7.3. Germany
7.3.1. Germany Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
7.3.2. Germany Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
7.4. France
7.4.1. France Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
7.4.2. France Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
7.5. Spain
7.5.1. Spain Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
7.5.2. Spain Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
7.6. Rest of Europe
7.6.1. Rest of Europe Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
7.6.2. Rest of Europe Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
7.7. Europe PEST Analysis
CHAPTER 8. Asia Pacific Virtual Clinical Trials Market By Country
8.1. Asia Pacific Virtual Clinical Trials Market Overview
8.2. China
8.2.1. China Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
8.2.2. China Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
8.3. Japan
8.3.1. Japan Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
8.3.2. Japan Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
8.4. India
8.4.1. India Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
8.4.2. India Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
8.5. Australia
8.5.1. Australia Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
8.5.2. Australia Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
8.6. South Korea
8.6.1. South Korea Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
8.6.2. South Korea Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
8.7. Rest of Asia-Pacific
8.7.1. Rest of Asia-Pacific Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
8.7.2. Rest of Asia-Pacific Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
8.8. Asia Pacific PEST Analysis
CHAPTER 9. Latin America Virtual Clinical Trials Market By Country
9.1. Latin America Virtual Clinical Trials Market Overview
9.2. Brazil
9.2.1. Brazil Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
9.2.2. Brazil Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
9.3. Mexico
9.3.1. Mexico Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
9.3.2. Mexico Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
9.4. Rest of Latin America
9.4.1. Rest of Latin America Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
9.4.2. Rest of Latin America Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
9.5. Latin America PEST Analysis
CHAPTER 10. Middle East & Africa Virtual Clinical Trials Market By Country
10.1. Middle East & Africa Virtual Clinical Trials Market Overview
10.2. GCC
10.2.1. GCC Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
10.2.2. GCC Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
10.3. South Africa
10.3.1. South Africa Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
10.3.2. South Africa Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
10.4. Rest of Middle East & Africa
10.4.1. Rest of Middle East & Africa Virtual Clinical Trials Revenue (USD Billion) and Forecast By Study Design, 2018-2030
10.4.2. Rest of Middle East & Africa Virtual Clinical Trials Revenue (USD Billion) and Forecast By Indication, 2018-2030
10.5. Middle East & Africa PEST Analysis
CHAPTER 11. Player Analysis Of Virtual Clinical Trials Market
11.1. Virtual Clinical Trials Market Company Share Analysis
11.2. Competition Matrix
11.2.1. Competitive Benchmarking of key players by price, presence, market share, and R&D investment
11.2.2. New Product Launches and Product Enhancements
11.2.3. Mergers And Acquisition In Global Virtual Clinical Trials Market
11.2.4. Partnership, Joint Ventures and Strategic Alliances/ Sales Agreements
CHAPTER 12. Company Profile
12.1. ICON plc
12.1.1. Company Snapshot
12.1.2. Business Overview
12.1.3. Financial Overview
12.1.3.1. Revenue (USD Million), 2021
12.1.3.2. ICON plc 2021 Virtual Clinical Trials Business Regional Distribution
12.1.4. Product /Service and Specification
12.1.5. Recent Developments & Business Strategy
12.2. Parexel International
12.3. IQVIA
12.4. Covance Inc.
12.5. PRA Health Sciences
12.6. LEO Innovation Lab
12.7. Medidata Solutions
12.8. Oracle Corporation
12.9. CRF Health
12.10. Clinical Ink, Inc.
12.11. Medable, Inc.
To Get Premium Report Full Copy in Form of Single user or Multiple user@https://www.acumenresearchandconsulting.com/buy-now/0/2614
About Us:
Acumen Research and Consulting (ARC) is a global provider of market intelligence and consulting services to information technology, investment, telecommunication, manufacturing, and consumer technology markets. ARC helps investment communities, IT professionals, and business executives to make fact based decisions on technology purchases and develop firm growth strategies to sustain market competition. With the team size of 100+ Analysts and collective industry experience of more than 200 years, Acumen Research and Consulting assures to deliver a combination of industry knowledge along with global and country level expertise.